 – health minister says probe points to gross inaccuracies by KN
– health minister says probe points to gross inaccuracies by KN
“I am satisfied with the manner in which the ministry is procuring its medical supplies, which are based on standards and regulations adopted from the WHO”
By Michael Younge

“Fictitious prices” are the words used by Health Minister Dr Bheri Ramsaran as he strongly dismissed and condemned the Kaieteur Newspaper for its continued misrepresentation and distortions of both the process through which government procures medical supplies and the prices it pays. Dr Ramsaran was at the time addressing a packed room of media operatives at the Office of the President on Wednesday, where he announced the findings of a probe that was launched into the allegations made by the newspaper against the NEW GPC INC.
“We have been able to look at the information regarding the specific products that would have been highlighted in certain sections of the media, more particularly, in the Kaieteur News,” the minister reported.
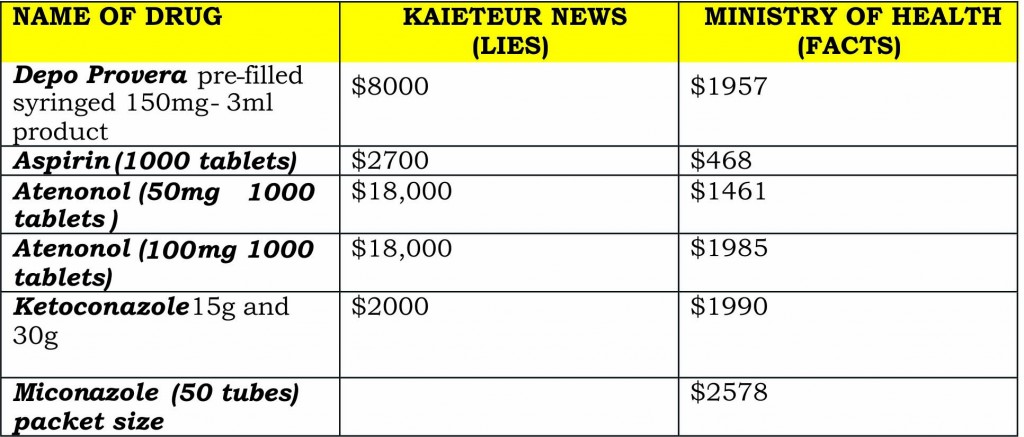
Depo Provera
Dr Ramsaran addressed what he called “the mischief of the myth of the pathological, spurious counterfeit claims of these inflated prices Gy$8000 versus a few hundred dollars” made by the Kaieteur News. Depo Provera, a contraceptive injection for females, was purchased for Gy$1561 in 2011 and not Gy$8000. This price for this pre-syringed 150mg to 3ml product has had a slight increase in 2012 to Gy$1957. The discredited Kaieteur News claimed that the Health Ministry had bought the injection for as much as Gy$8 000 from the NEW GPC.
Aspirin
“A packet size of 1000 aspirin tablets with a dosage of 300 milligrams we paid Gy$485 and for some reason this year it seems to have dropped to Gy$468… whereas, the claim or the fictitious price claimed for the two particular years were Gy$2700… those are some discrepancies,” Dr Ramsaran remarked.
Atenonol
Atenonol, a frequently used drug – the claim was that the ministry was spending Gy$18,000 on a 1000 package size of this product. In 2011, the price paid was Gy$1165 and in 2012, Gy$1461, for the 50 mg tablets in 1000 package. For 100 mg, the ministry paid in 2011, Gy$1693 and this year Gy$1985.
Ketoconazole and miconazole
Dr Ramsaran also noted that with respect to the issue of skin creams, while the prices were Gy$1990 for ketoconazole and Gy$ 2578 for miconazole (50 tubes). The health minister used the information published by the Kaieteur News as the sole basis for his investigation, and did a comparison with the prices that the items were purchased for by the Georgetown Public Hospital Corporation (GPHC) and the Health Ministry.
He was convinced that the newspaper was up to sheer mischief as it continued to launch attacks back and forth against the NEW GPC and the Health Ministry.
Dr Ramsaran said that he was “satisfied” with the manner in which the ministry was procuring its medical supplies, explaining that the standards and regulations in place were adopted from the World Health Organisation.
He said that his government remained committed towards ensuring that there are no health risks to Guyanese, noting that a series of stringent requirements have to be satisfied when procuring medical supplies.
He lauded the prequalification process even in the midst of continued pressure from the media about the overall tendering process, award of contracts and the complaints made by the International Pharmaceutical Agency.
The health minister said the process and guidelines were serving their purpose as the country has done well in trying to alleviate the presence of counterfeit drugs on the market. “Substandard, spurious, falsely label, falsified, counterfeit drugs” was a major issue that engaged the attention of the World Health Assembly recently, he noted, stating again that the prequalification process, the need for checks and balances and several systems that Guyana and other developing countries follow have been lauded and recommended.
“We were warned that under every bottom house you can find a pharmacy plant in the developing world, so there was need for caution,” Dr Bheri explained.
“I am satisfied that the tendering process was followed as stipulated in the laws,” he remarked, noting that it was not the government of Guyana or the Health Ministry, much less the GPHC that was responsible for the selection, tendering process or evaluation of bids.
“There are divisions of function,” he argued, pointing out that it was the responsibility of the National Procurement and Tender Board to handle this task.
The board only approved two companies as prequalified to supply the Health Ministry with medical supplies. The International Pharmaceutical Agency is not one of these companies. Dr Ramsaran said that the firm could get all the answers it wants from the board about its disqualification, as he explained that this could not be a draconian task.
“I think by law we are constrained to do that… I see no difficulty with the extract of the evaluation report in some way becoming available.”
Meanwhile, the issue of inventory management to deal with expired drugs was also addressed by the health minister.
Expired drugs
Systems are being improved at the Materials Management Unit (MMU) and a change management scheme consultancy has over the past few years been installing software and other systems and training staff in the central bond and other major locations. Dr Ramsaran did note that this only accounts for about 80 per cent to 85 per cent of distributed quantities of pharmaceuticals. “There are still drugs expiring… we are aiming for a first in and first out process,” the health minister added, as he addressed the issue which has been a concern.
New bond
There are plans to start from scratch with a new bond in Diamond. This will be under a transitional management, and issues will be dealt with including drugs expiring, in addition to medical equipment. “We have not costed those drugs that are expiring… we moving our new warehouse, is by the Busta sign in Diamond… it will be a military operation… we are engaging the AG so that when we moving, there is no slippages or disappearance of medical supplies…” he explained.
Materials stored from the old bond at Farm, East Bank Demerara, will not be decanted into the new facility. The issue of requisition for drugs, the forms and their preparation in a timely manner will be addressed, focusing on human errors and disciplinary methods.




Comments are closed.